Copper was discovered by Neolithic Man about 9000 years ago and used in place of stone, as it was far easier to shape. Early coppersmiths in Iran found that heating copper softened it, and hammering copper made it harder. This way, they could shape copper into valuable items such as containers and utensils, a big leap forward for the human race. [1]
Lee, J., Bazilian, M., Sovacool, B., & Greene, S. (2020). Responsible or reckless? A critical review of the environmental and climate assessments of mineral supply chains. Environmental Research Letters, 15(10), 103009. https://doi.org/10.1088/1748-9326/ab9f8c
Copper and its usage
Copper is known for excellent conductivity, corrosion resistance, strength, and malleability, making it ideal for various industrial applications such as [2]:
- Electrical and electronic industry: Copper is widely used to produce electrical wires, cables, and components such as circuit boards and connectors.
- Construction industry: Copper is utilized in constructing buildings, bridges, and infrastructure due to its strength, durability, and corrosion resistance.
- Plumbing and sanitary ware: Copper pipes and fittings are used in plumbing systems to resist corrosion and high heat conductivity.
- Heating, Ventilation, and Air Conditioning (HVAC) and refrigeration: Copper is employed in heat exchangers, condensers, and evaporators for its high heat transfer efficiency and corrosion resistance.
- Automotive industry: Copper is used in various automotive components, such as engine blocks, exhaust, and electrical systems.
- Consumer products: Copper can be found in everyday items like kitchen appliances, lighting fixtures, and musical instruments.
- Agriculture: Copper is used in various agricultural applications, such as fungicides and bactericides, to protect crops from pests and diseases.
- Photovoltaics: Copper is used in producing solar panels due to its high conductivity and durability.
- Batteries: Copper is a key component in producing batteries, particularly in the cathodes of lithium-ion batteries.
- Antimicrobial applications: Copper’s antimicrobial properties are used in products like copper-infused textiles, which can help prevent the spread of bacteria and viruses.
How copper is created?
Because copper reacts readily with other substances, it can be formed in various ways in the Earth’s crust. It is often found in deposits with metals such as lead, zinc, gold and silver. The most significant amounts of copper are in the crust as porphyry copper deposits. [1].
One of the main geological processes through which copper is formed is magmatic processes, where copper is concentrated in magma chambers and then transported to the Earth’s surface through volcanic activity. Porphyry copper deposits, the world’s largest source of copper, are formed through this process [3]. Another way copper is formed is through hydrothermal processes, where hot fluids circulate through rocks and deposit copper minerals. This process can occur in various geological settings, including volcanic arcs, rift zones, and sedimentary basins [4].
Additionally, copper can be formed through microbial processes, where microorganisms mediate the formation of copper sulfide nanoparticles via organic sulfur mineralization in soil [5]. Copper can also be found in various anthropogenic sources, including industrial discharges, sewage treatment plants, surface runoff, septic tanks, agriculture, mariculture, marine transport, copper-based plant protection products, and farmyard manure. [6,7,8,9].
Copper production
The global production of copper has been significant, with the extraction of copper reaching 20.2 million tons in 2016, while the output from metallurgical plants attained 19.0 million tons in the same year. Refined copper production output increased to 23.3 million tons in 2016, including 3.9 million tons from recycling. Notably, there has been a fast increase in copper production in South America, particularly in Chile and Peru, which accounted for 41% of the global copper production. [10] In descending order, the ten leading countries in the world copper production as of 2022 were Chile, Peru, the Democratic Republic of the Congo, China, the United States, Russia, Indonesia, Australia, Zambia, and Mexico. Chile, the world’s leading copper producer, produced an estimated 5.2 million metric tons of copper in 2022. Peru and the Democratic Republic of the Congo were second, with an estimated copper mine production of 2.2 million metric tons in the same year. China is the world’s third-largest copper producer from mines, with an estimated 1.9 million metric tons of copper from mines. [11]
According to a report by McKinsey, Southeast Asia is a top producer of base metals, including nickel and copper, and the region has seen an increase in commodity prices for base metals such as copper [12]. Additionally, a Reuters article highlights that Southeast Asia and India are expected to account for 20% of global refined copper demand growth from 2023 to 2028 [2]. This indicates a growing demand for copper in the region, driven by new energy initiatives and industrial development.
Copper mining
Two of the most common copper ores are copper oxide and copper sulfide. Copper oxides are more abundant near the surface but are considered low-grade ore. On the other hand, copper sulfide is less abundant but is regarded as high-grade ore. [13] Copper is mined through a multi-step process [14].
Copper mining, especially for lower-grade ores, is usually performed using open-pit mining, in which a series of stepped benches are dug deeper and deeper into the earth over time. Boring machinery is used to drill holes into the hard rock to remove the ore, and explosives are inserted into the drill holes to blast and break the rock. The resulting boulders are then ready for hauling from the blasting site to the processing site. The equipment needed to haul the tons of ore is gigantic. Most ores are sent through a primary crusher, typically located close to or sometimes in the pit. This primary crusher reduces the size of the ore from boulders to golf ball-sized rocks. In underground copper mining, vertical shafts are sunk well over 1,000 meters below the surface, and tunnels are extended to the ore body. The ore, broken by drilling and blasting, is hoisted through the shaft and conveyed to the processing plant. [13,15]
The copper oxide and copper sulfide undergo two different extraction processes, hydrometallurgy and pyrometallurgy, respectively, due to their different chemistries.
Hydrometallurgy uses aqueous (water-based) solutions to extract and purify copper from copper oxide ores at ordinary temperatures, usually in three steps: heap leaching, solvent extraction, and electrowinning.
Heap leaching: is the process of using percolating chemical solutions to leach out metals [15]. The crushed copper ore is stacked on an impermeable pad, and a leaching reagent or a strong acid, commonly sulfuric acid for copper ores, is added by irrigation from the top. The copper mineral is extracted, and the solution from this, known as pregnant leach solution (PLS), is collected at the base of the heap. The PLS is then pumped for further copper extraction processing. [16]Solvent extraction: is the process when two immiscible liquids are stirred and allowed to separate, causing the copper to move from one liquid to another. The PLS is mixed vigorously with a solvent, commonly an organic solvent, that selects the copper ion. When a copper ion in the PLS is extracted, an H+ ion from the organic solvent replaces the copper ion in the solution. This exchange allows the leaching acid to return to its original pH and enables the acid solution to be re-used for ore leaching in the heap leaching process. [15, 16]Electrowinning: is a type of electrolysis. An electrical current passes through an inert anode (positive electrode) and through the copper solution from the solvent extraction process, which acts as an electrolyte. Positively charged copper ions (cations) come from the solution and are plated onto a cathode (negative electrode) as 99.99% pure copper. [16]
Pyrometallurgy uses heat to extract and purify copper from copper sulfide ore and concentrates in four steps: froth flotation, thickening, smelting, and electrolysis.
Froth flotation: is the process in which the finely ground copper ore is mixed with water and certain chemicals, such as collectors, to make the copper particles water-repellent. Air is then bubbled through the mixture, causing the copper minerals to attach to the air bubbles and rise to the surface as a froth, while the gangue (unwanted material) sinks to the bottom. The froth containing the copper minerals is then collected and further processed. [16]Thickening: The froth is poured into large tanks called thickeners. The bubbles broke, and solids from the froth solution settled at the bottom of the tank. The solids are then filtered to remove excess water, which can be reused to process additional sulfide ore batches. [16]Smelting: Typical smelting process includes roasting, smelting, concentrating, and fire refining. The roasting process is to dry and heat the incoming feed producing a “calcine”. The smelting process is when the calcine is melted with siliceous flux in a smelting furnace to produce copper matte. The copper matte is taken to another furnace called a converter to have the remaining iron and sulfur burned off; the product is called blister copper. The blister copper is further burned off and poured into molds called anode-casting wheels. The cooled anode slabs are 99% pure copper. [15]Electrolysis: is the final process of purifying sulfide ore into copper cathodes. The copper anode slab and pure cathode are placed into a tank filled with copper sulfate and sulfuric acid electrolyte. An electric current is applied so positively charged copper ions leave the anode (positive electrode) and move in solution through the electrolyte solution to be plated on the cathode (negative electrode). Other metals and impurities also leave the anode. The electrolysis can result in 99.99% pure copper. [15]
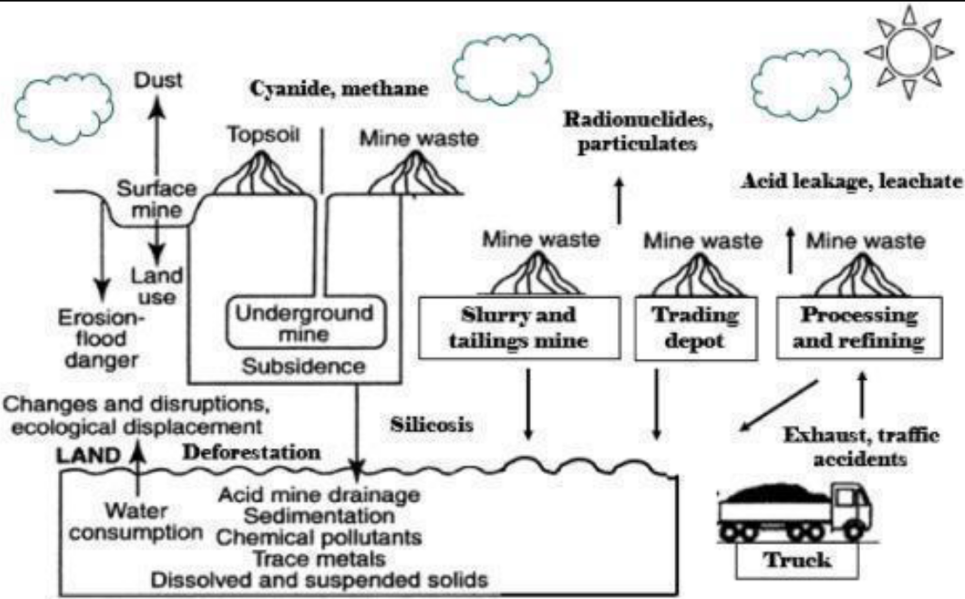
Impacts of copper mining
- Gaseous Emissions and Particulate Matter: The copper production is estimated to account for approximately 0.2% to 0.3% of global Greenhouse Gas (GHG) emissions, with the potential to increase as demand for copper rises [17, 18, 19]. The emissions are mainly caused by fuel combustion in mining equipment, electricity consumption, and the processing of low-grade copper ore, increasing the production’s carbon intensity [18, 20, 21]. The increasing energy demand to produce the same amount of copper is a notable factor contributing to rising emissions. As the demand for copper is expected to double by 2050, efforts to mitigate GHG emissions from copper mining are crucial [18]. Various studies and reports emphasize the need for transparency and the development of consistent approaches to measure and reduce the carbon footprint of copper production. [19, 20, 22] In addition, copper mining also has concerns about particulate matter. The sulfide-ore copper mining and the by-products from mining and metal processing, such as ashes, slags, and dust, are sources of toxic metals, such as Pb, Cd, Hg, As, Al, and particulate matter [23]. The uncontrolled copper smelting processes will emit large quantities of particulate matter, trace elements, and sulfur [24].
- Dumping and Hazardous Solid Waste: The disposal of hazardous solid waste from copper mining, such as copper flotation waste, mine drainage water, and by-products from mining and metal processing, poses significant environmental and human health risks. These wastes contain toxic metals such as Pb, Cd, Hg, As, and Al, as well as high levels of particulate matter. The geochemical analysis of mining waste has shown elevated concentrations of these hazardous elements, which can threaten the environment and human health if improperly disposed of. The potential impacts include water and soil pollution, long-distance transportation of heavy metals, and the creation of smog. [23, 25, 26]
- Water Consumption: Copper extraction typically demands vast quantities of fresh water, with an estimated 5,300 gigalitres of water used in 2016 [27]. A conventional copper processing plant uses between 0.45 and 0.6 m³ of water per dry tonne of ore, which can amount to around 30,000 cubic meters of fresh water daily for a 50,000 tons per day ore copper mine [28]. Two main factors drive increased water consumption in the copper industry: changes in process routes (e.g., from solvent extraction and ionic withdrawal to flotation) and a decline in ore grades. [29]
- Electricity Consumption: The specific electricity required per ton of produced copper is about 12.0 GJ/t of copper in both the concentrator and the Leach Solvent Extraction Electrowinning (LS-SX-EW) processes, with the value having increased at a faster overall rate over the past 10 years for plants equipped with concentrators [30]. In addition, the copper smelter and refinery plants use approximately 200 gigawatt-hours (GWh) of electric power annually [31]. The expected electricity consumption in Chilean copper mining is forecasted to increase from 21.1 Terawatt-hour (TWh) to 29.2 TWh, with an average yearly growth rate of approximately 2.7% [32]. Furthermore, all aspects of copper production require energy, with approximately 36.1% of the energy in electricity [33]. The operations of rear dump trucks accounted for the majority of energy consumed in copper mining, representing 66% of the total energy consumed [34].
- Land Use and Landscape Degradation: Copper mining can significantly impact land use and landscape degradation. Deforestation is one of the main issues associated with mining activity, as large areas of natural habitat are destroyed during mine construction and exploitation, forcing animals to leave the site. Open-pit mines can be nearly a mile in diameter and several thousand feet deep, requiring miners to remove significant amounts of forest, home to thousands of wildlife species. Animals can be poisoned directly by mine products and residuals, and bioaccumulation in the plants or the smaller organisms they eat can also lead to poisoning. Disposing of copper mining leaching solutions via land application or other means can also cause land degradation. [35,36]
By: Hendra WINASTU, SOLEN Principal Associate – IPC panel coordinator
Edited by: Nguyeng Duy Hung, SOLEN Director – IPC program director
Date: 15 January 2024
Article#: SOLEN-IPC-0031
Ref:
[1] Australia, G. (2023, September 7). Copper. Geoscience Australia. https://www.ga.gov.au/education/minerals-energy/australian-mineral-facts/copper [2] Wu, S., He, S., Yang, Y., Lin, Y., Chang, T., Peng, C.Y., & Hsieh, M. (2020). Research on Environmentally Friendly Chemical Technology for Green Reusable and Sustainable Water Metal Copper Ions. IOP Conference Series: Earth and Environmental Science, 555. [3] Steinberger, I.T., Hinks, D.R., Driesner, T., & Heinrich, C.A. (2013). Source Plutons Driving Porphyry Copper Ore Formation: Combining Geomagnetic Data, Thermal Constraints, and Chemical Mass Balance to Quantify the Magma Chamber Beneath the Bingham Canyon Deposit. Economic Geology, 108, 605-624. [4] Park, J., Campbell, I.H., Chiaradia, M., Hao, H., & Lee, C.A. (2021). Crustal magmatic controls on the formation of porphyry copper deposits. Nature Reviews Earth & Environment, 2, 542 – 557. [5] Xu, H., Zhang, P., He, E., Peijnenburg, W.J., Cao, X., Zhao, L., Xu, X., & Qiu, H. (2023). Natural formation of copper sulfide nanoparticles via microbially mediated organic sulfur mineralization in soil: Processes and mechanisms. Geoderma. [6] Shotyk, W. (2020). Natural and anthropogenic sources of copper to organic soils: a global, geochemical perspective1. Canadian Journal of Soil Science, 100, 516 – 536. [7] Comber, S., Deviller, G., Wilson, I., Peters, A., Merrington, G., Borrelli, P., & Baken, S. (2023). Sources of copper into the European aquatic environment. Integrated environmental assessment and management, 19(4), 1031–1047. https://doi.org/10.1002/ieam.4700 [8] Wang, Z., Hong, C., Xing, Y., Wang, K., Li, Y., Feng, L., & Ma, S. (2018). Spatial distribution and sources of heavy metals in natural pasture soil around copper-molybdenum mine in Northeast China. Ecotoxicology and environmental safety, 154, 329–336. https://doi.org/10.1016/j.ecoenv.2018.02.048 [9] Panagos, P., Ballabio, C., Lugato, E., Jones, A., Borrelli, P., Scarpa, S., Orgiazzi, A., & Montanarella, L. (2018). Potential Sources of Anthropogenic Copper Inputs to European Agricultural Soils. Sustainability. [10] Pietrzyk, S., & Tora, B. (2018). Trends in global copper mining – a review. IOP Conference Series: Materials Science and Engineering, 427. [11] Statista. (2010). Global copper production (by country) 2010-2018 | Statista. Statista; Statista. https://www.statista.com/statistics/264626/copper-production-by-country/ [12] Optimizing processes in Southeast Asia’s mining industry | McKinsey. (n.d.). Www.mckinsey.com. https://www.mckinsey.com/industries/metals-and-mining/our-insights/advancing-metals-and-mining-in-southeast-asia-with-digital-and-analytics [13] Copper processing – Ores. (n.d.). Encyclopedia Britannica. https://www.britannica.com/technology/copper-processing/Ores [14] Derpich, I., Muñoz, N., & Espinoza, A. (2019). Improving the productivity of the copper mining process in the Chilean copper industry. Croatian Operational Research Review, 227-240. [15] The University of Arizona. (2020, July 13). Copper Mining and Processing: Processing Copper Ores. Superfund. https://superfund.arizona.edu/resources/learning-modules-english/copper-mining-and-processing/processing-copper-ores [16] Copper Heap Leaching. (n.d.). Encyclopedia.pub. https://encyclopedia.pub/entry/14992 [17] Watari, T., Northey, S., Giurco, D., Hata, S., Yokoi, R., Nansai, K., & Nakajima, K. (2022). Global copper cycles and greenhouse gas emissions in a 1.5 °C world. Resources, Conservation and Recycling, 179, 106118. https://doi.org/10.1016/j.resconrec.2021.106118. [18] Understand your copper emissions. (n.d.). Www.carbonchain.com. https://www.carbonchain.com/blog/understand-your-copper-emissions [19] Carbon Footprint of Copper Production: Best Practice Guidance for Greenhouse Gas Measurements – Copper Alliance. (n.d.). Https://Copperalliance.org/. https://copperalliance.org/resource/carbon-footprint-of-copper-production-best-practice-guidance-for-greenhouse-gas-measurements/ [20] Azadi, M., Northey, S. A., Ali, S. H., & Edraki, M. (2020). Transparency on greenhouse gas emissions from mining to enable climate change mitigation. Nature Geoscience, 13(2), 100–104. https://doi.org/10.1038/s41561-020-0531-3 [21] Study: Copper Mining and Climate Change. (2021, February 9). Friends-Bwca.org. https://www.friends-bwca.org/blog/climate-change/Prospective Strategies to Improve the Environmental Sustainability of the Copper Mining Industry in Peru. (2023). https://doi.org/10.46254/na07.20220430 [22] Emerman, S.H. (2020). Testimony before House Natural Resources Subcommittee on Indigenous Peoples of the United States Hearing on “The Irreparable Environmental and Cultural Impacts of the Proposed Resolution Copper Mining Operation”. [23] Izydorczyk, G., Mikula, K., Skrzypczak, D., Moustakas, K., Witek-Krowiak, A., & Chojnacka, K. (2021). Potential environmental pollution from copper metallurgy and methods of management. Environmental Research, 197, 111050. https://doi.org/10.1016/j.envres.2021.111050 [24] Environmental Aspects of Copper Production. (n.d.). https://www.princeton.edu/~ota/disk2/1988/8808/880810.PDF [25] US EPA, O. (2015, April 22). TENORM: Copper Mining and Production Wastes. US EPA. https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes [26] Covre, W. P., Ramos, S. J., Pereira, W. V. da S., Souza, E. S. de, Martins, G. C., Teixeira, O. M. M., Amarante, C. B. do, Dias, Y. N., & Fernandes, A. R. (2022). Impact of copper mining wastes in the Amazon: Properties and risks to the environment and human health. Journal of Hazardous Materials, 421, 126688. https://doi.org/10.1016/j.jhazmat.2021.126688


 Tiếng Việt
Tiếng Việt 日本語
日本語