Wetlands are often home to many species of microorganisms and plants. The ideal physical and chemical conditions found in wet soils have created a diversity of organisms from the smallest micro-organisms to the largest plants. This biodiversity creates interactions between species, thereby making better use of energy flows and providing superior efficiency in the natural processes of wetland ecosystems. Wetlands designed to treat wastewater based on biological activity result in lower initial investment costs, lower operating and maintenance costs, and more consistent system performance.
The following article will present the ecology of wetlands applied in wastewater treatment.
PART 1: BACTERIA AND FUNGI IN WETLANDS
Wetlands and aquatic habitats provide suitable conditions for the growth and reproduction of microorganisms. Two important groups of microorganisms are bacteria and fungi. These microorganisms play a very important role in wetland wastewater treatment systems mainly due to their role in assimilation, transformation and reuse of chemical constituents present in various types of wastewater. different wastewater.
The taxonomy of bacteria in wetlands is complex and frequently changing, but generally recognized groups of bacteria and fungi exist in this environment. Bacteria are classified as prokaryotes. Fungi are classified as eukaryotes because they have a nucleus separated from the cytoplasm by a nuclear membrane.
1. Bacteria
Bacteria are single-celled, prokaryotic organisms classified according to morphology, chemical, nutritional, and metabolic characteristics. Bergey’s Manual classifies bacteria into 19 groups linked to evolutionary relationships. Most bacteria can be classified according to four morphologies: cocci, bacilli, spirochetes, and filaments. These organisms can grow singly or in groups of cells they associate into pairs, chains of colonies. Bacteria usually reproduce by cell division, in which cells divide into two equal daughter cells.
Most bacteria are heterotrophic, which means they have their nutritional and energy requirements to grow from organic compounds. In addition, some autotrophic bacteria synthesize organic molecules from inorganic carbon sources (carbon dioxide, CO2).
Some bacteria cannot move on their own while others move using flagella. In wetlands, most bacteria are present on the solid surfaces of plants, decaying organic matter, and soil.
2. Mushrooms
Fungi represent a group of eukaryotic organisms, including yeasts, molds, and fruity fungi. All fungi are heterotrophic, i.e. have an energy requirement and a source of organic carbon. Most of the nutritional pathways of fungi are saprophytic, based on the breakdown of dead organic matter. Fungi are abundant in wetland environments and play an important role in water treatment.
Fungi are ecologically important in wetlands because they mediate the reuse of carbon (a symbiotic process of bacteria and algae) and other nutrients in wetland environments. After the microbial action, the vegetation becomes rotting and becomes a medium for aquatic fungi to grow and through saprophylaxis, dead organic matter is decomposed.
Fungi live in symbiosis with algae (lichens) and higher plants (mycorhizzae), increasing the efficiency of the host’s uptake of nutrients from the air, water, and soil. If fungi are inhibited by the effects of toxic metals and other chemicals in the wetland environment, the breakdown of nutrients can be reduced, significantly limiting the algae’s ability to treat and higher plants. In wetlands, fungi are often found on dead and decaying vegetation.
3. Biological metabolism of microorganisms
Most of the important chemical changes carried out by bacteria are controlled by enzymes, which are specific proteins that catalyze chemical reactions. To varying degrees, bacteria and fungi are classified according to their ability to catalyze certain reactions. Microbial metabolism involves the use of enzymes to break down complex organic compounds into simpler ones along with the release of energy or synthesis of organic compounds using chemical storage of energy.
The metabolism of microorganisms depends not only on the presence of suitable enzymes but also on environmental conditions such as temperature, dissolved oxygen (DO) and hydrogen ion concentration (pH). . In addition, the concentration of the chemical substrate through the transformation plays an important role in determining the reaction rate.
Microbial metabolism
During microbial metabolism, carbohydrates are broken down to pyruvic acid with the production of two molecules of pyruvic acid and two molecules of adenosine triphosphate (ATP) for each molecule of glucose and the subsequent breakdown of pyruvic acid. through fermentation or respiration.
– Fermentation by phosphorylation at substrate conditions without oxygen, forming a variety of organic end products such as lactic acid, ethanol and other organic acids.
Aerobic respiration is a biochemical reaction by which carbohydrates are broken down into CO2 , water and energy (38 ATP molecules for each glucose molecule fully oxidized). The Krebs cycle results in the loss of carbon dioxide (decarboxylation) and energy storage (two ATP molecules per glucose molecule). The general response for aerobic respiration can be summarized as follows:
C 6 H 12 O 6 +6H 2 O+6O 2 +38 ADP +38 P = 6CO 2 +12H 2 O+38 ATP
In addition, about 60% of the energy of the original glucose molecule is lost as heat during complete aerobic respiration.
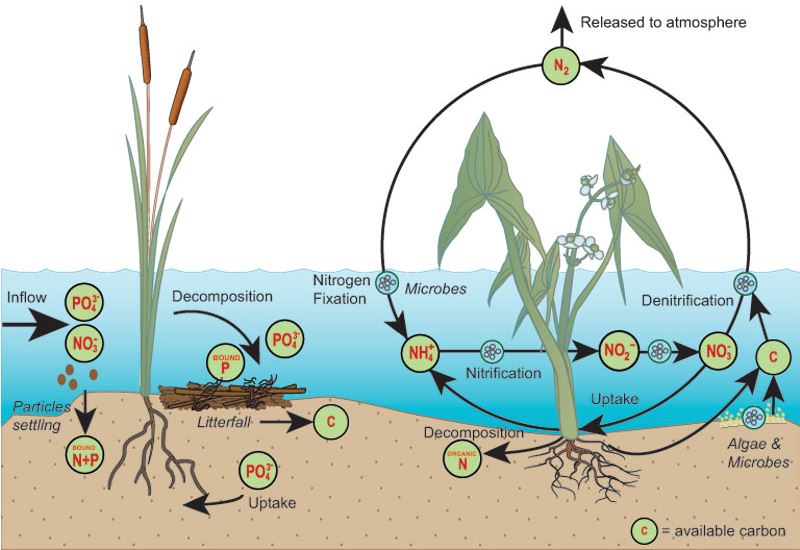
Anaerobic respiration is an alternative catabolic process that occurs in the absence of free oxygen. In anaerobic respiration, several other inorganic compounds are used as final electron acceptors. Anaerobic respiration produces a lower amount of energy. This form of respiration is important for certain groups of bacteria that occur in wetlands and aquatic habitats. Bacteria of the genera Pseudomonas and Bacillus use nitrate nitrogen as the final electron acceptor, producing nitrite, nitrous oxide (N 2 O) or nitrogen gas (N 2 ) by denitrification. The bacteria Desulfovibrio uses sulfate (SO 4 2- ) as an electron acceptor leading to the formation of H 2 S. Methanobacterium uses carbonate (CO 3 2- )) form methane gas (CH 4 ).



 Tiếng Việt
Tiếng Việt 日本語
日本語